Key Results from Study
New study describes a functional, aged, human cell-based platform that examines the contribution of senescence in Alzheimer’s Disease progression, and response to therapeutics.
- Senescence role in AD established using a long-term potentiation assay combined with biomarkers
- Human-on-a-Chip® platform models cognitive aging to evaluate therapeutics
ORLANDO, FLORIDA - Researchers from Hesperos and the University of Central Florida have developed a
groundbreaking model using human induced pluripotent stem cell (iPSC)-derived cortical neurons to better
understand and combat Alzheimer’s disease (AD). Their study, “A functional aged human iPSC-cortical neuron model recapitulates Alzheimer’s disease, senescence, and the response to therapeutics," has been published in
the prestigious journal Alzheimer's & Dementia ® : The Journal of the Alzheimer's Association. doi.org/10.1002/alz.14044
AD affects an estimated 6.2 million Americans aged 65 and older, with projections suggesting this number could reach 14 million by 2050. Current treatments, such as acetylcholinesterase inhibitors and NMDA receptor antagonists, provide only temporary relief. The high failure rate of AD clinical trials highlights the need for a better understanding of the pathophysiology and drug mechanisms.
The study addresses a critical challenge in Alzheimer’s research: the degeneration of cortical layers associated with cognitive decline. Despite substantial efforts to date, few current AD therapies are disease-modifying. The team accurately reproduced key biological aspects of aging and senescence in a multi-cellular microphysiological system (MPS), also known as a Human-on-a-Chip. This new approach reveals how cellular aging, also known as senescence, contributes to disease progression and therapy resistance, providing researchers with an accurate, human platform to further understand the disease and potential therapeutics in a living context.
Further, since other platforms at Hesperos, based on neurodegenerative diseases, have been accepted by the Food and Drug Administration (FDA) for efficacy data, it provides a new method to accelerate development and regulatory submissions of new treatments.
This model provides an invaluable tool for real-time measurement of neuronal activity and aging biomarkers, facilitating drug screening and the development of AD therapies. It also reveals complex disease mechanisms, such as the role of inflammatory factors in neuronal senescence and how it differs from non-disease age-related controls, offering new targets for intervention.
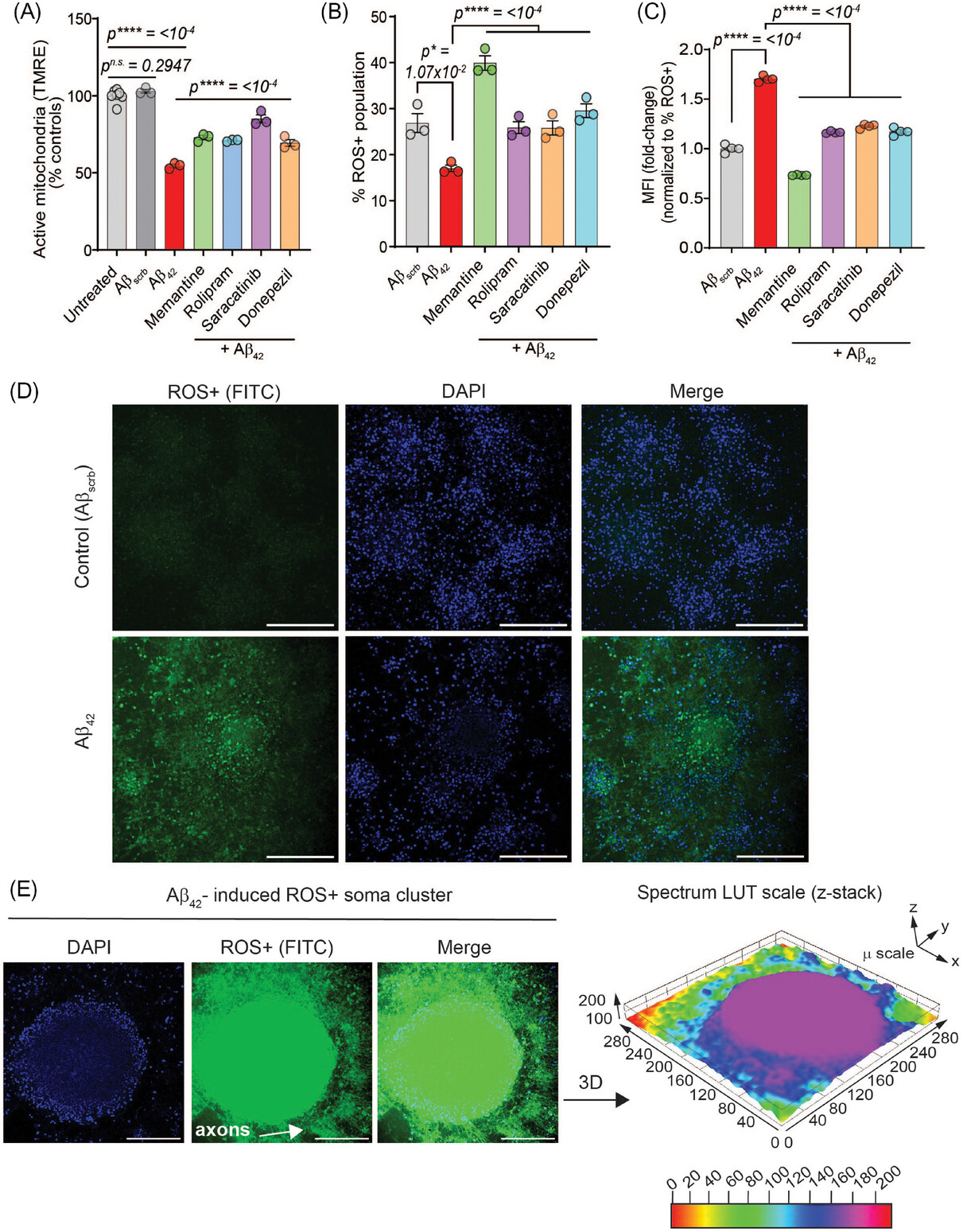
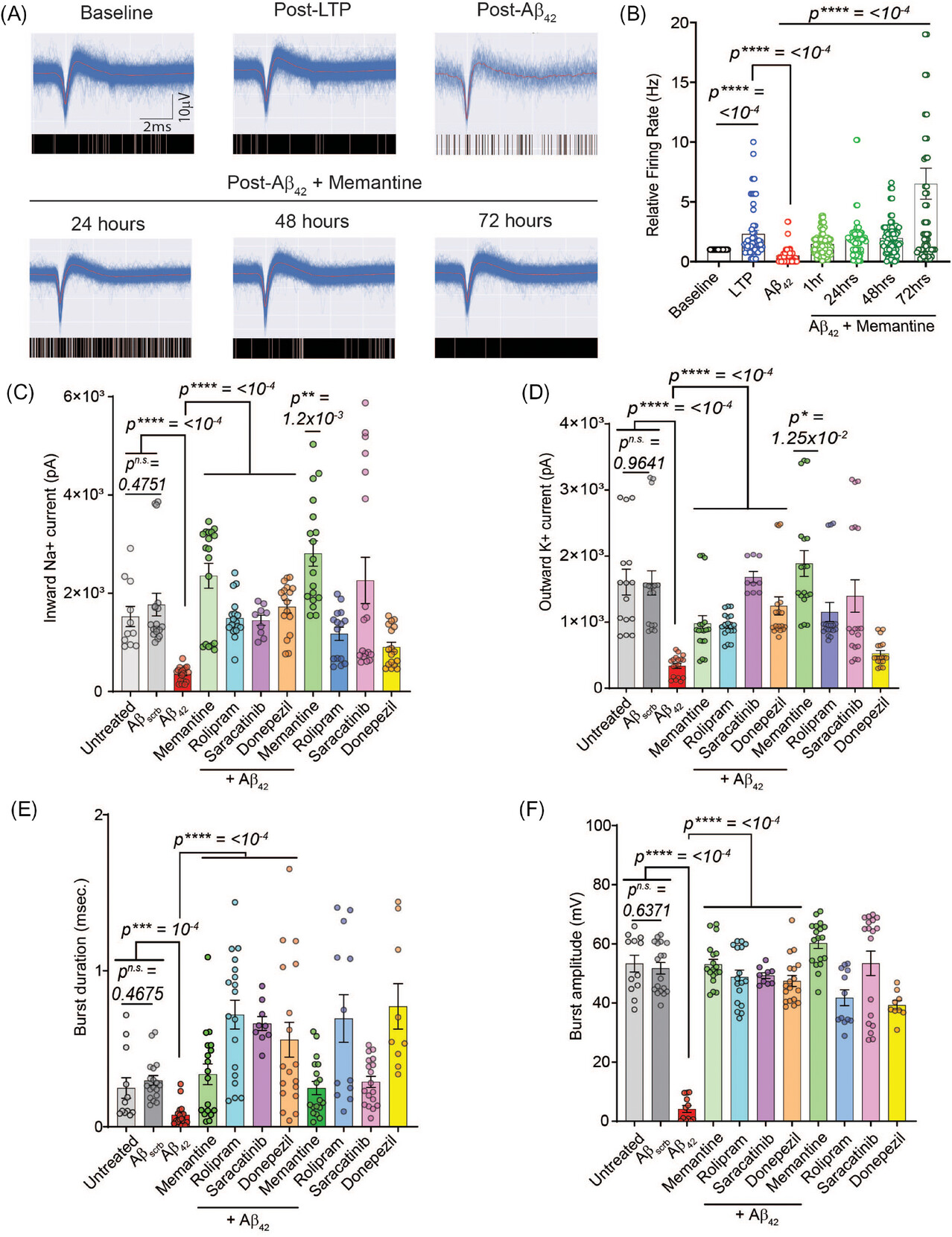
Researchers cultured human iPSC-derived cortical neurons on patterned microelectrode arrays to measure long-term potentiation (LTP) noninvasively. LTP, a quantifiable metric for learning and memory, is directly impacted by central nervous system neurodegeneration. By treating these neurons with pathogenic amyloid-β (Aβ), a peptide that plays a central role in development of AD, to mimic disease pathophysiology, the team was able to analyze senescence and therapeutic responses.
The study demonstrates that this novel human iPSC-cortical neuron model of aging, cultured in serum-free, defined conditions, accurately recapitulates hallmarks of AD showing the following:
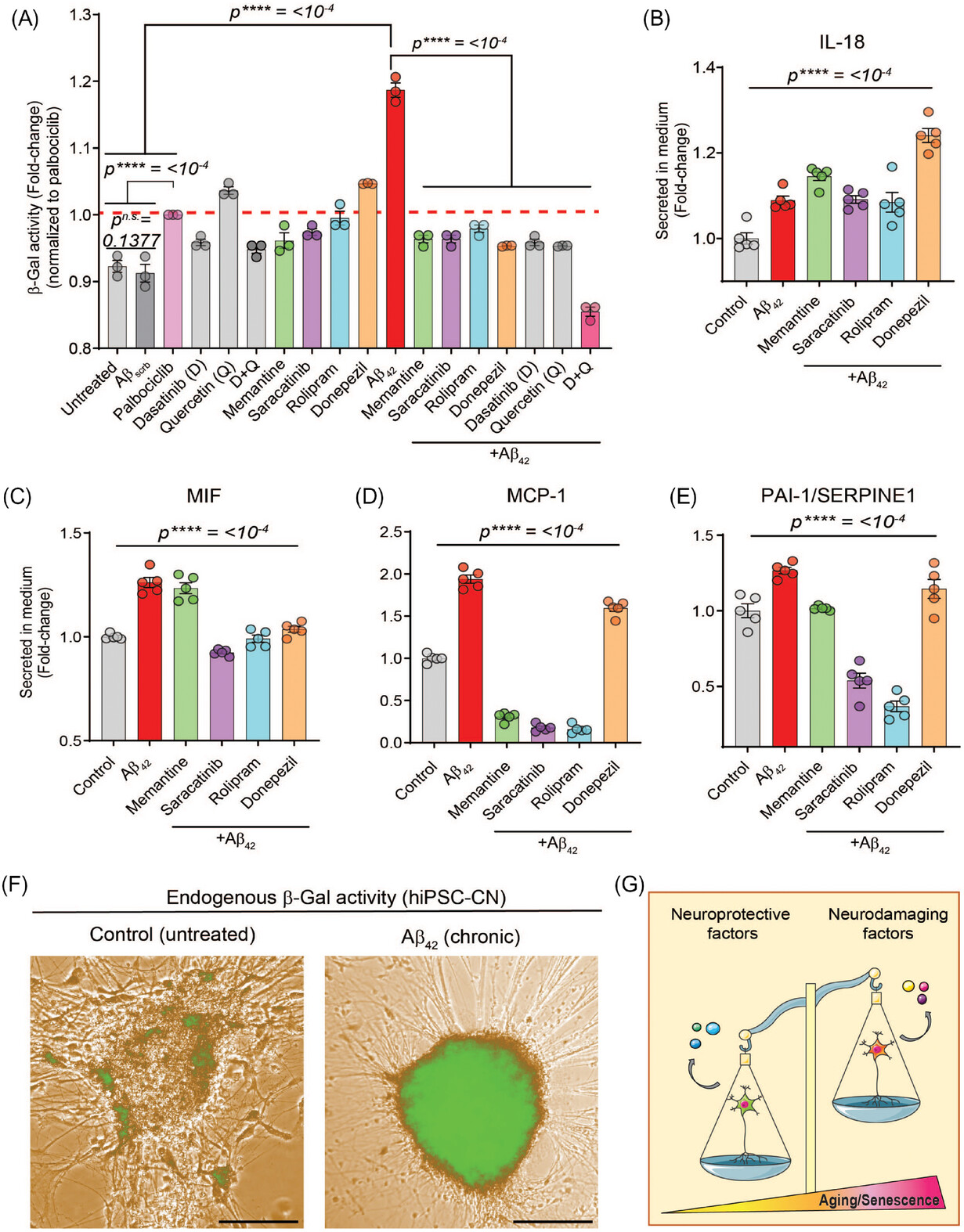
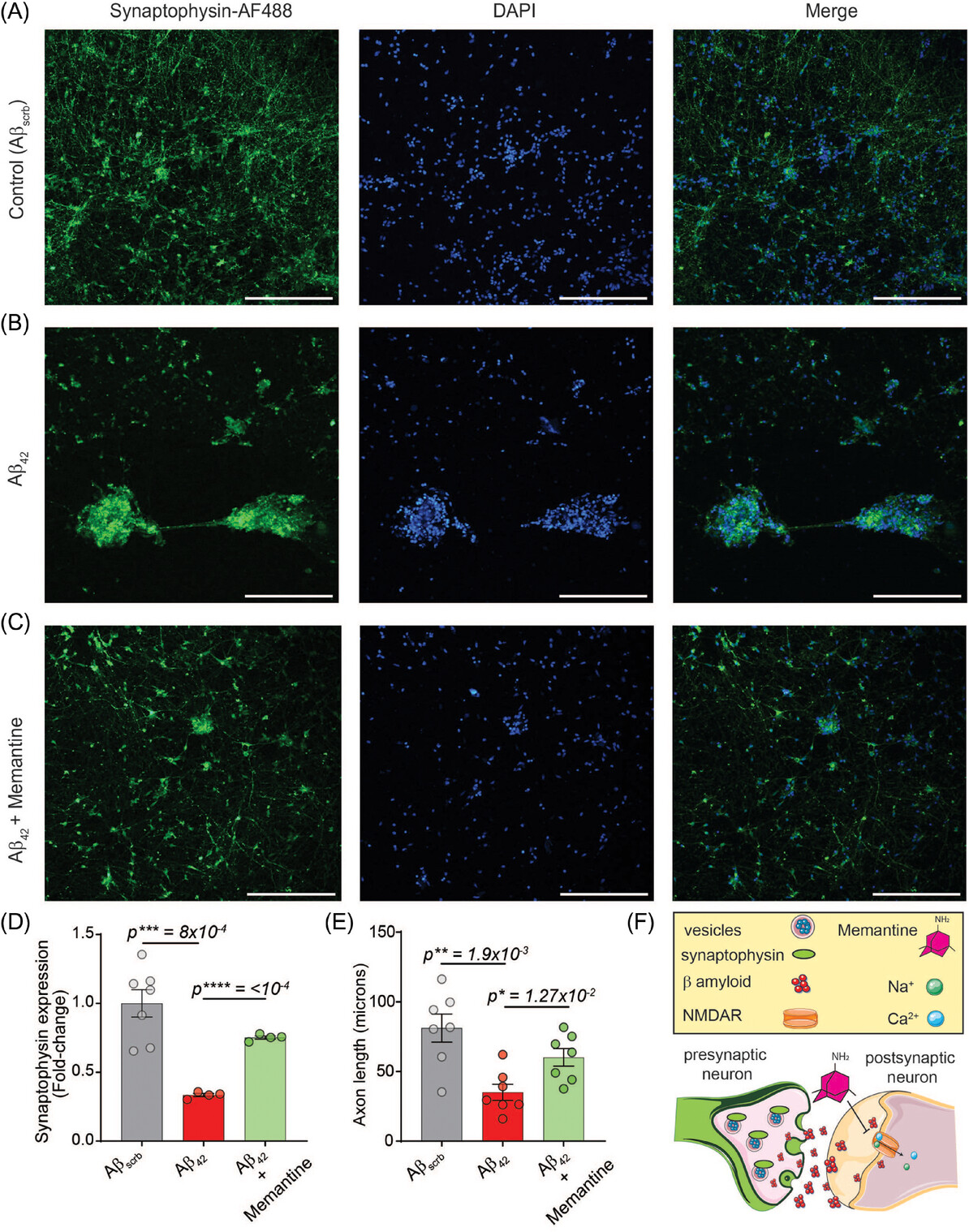
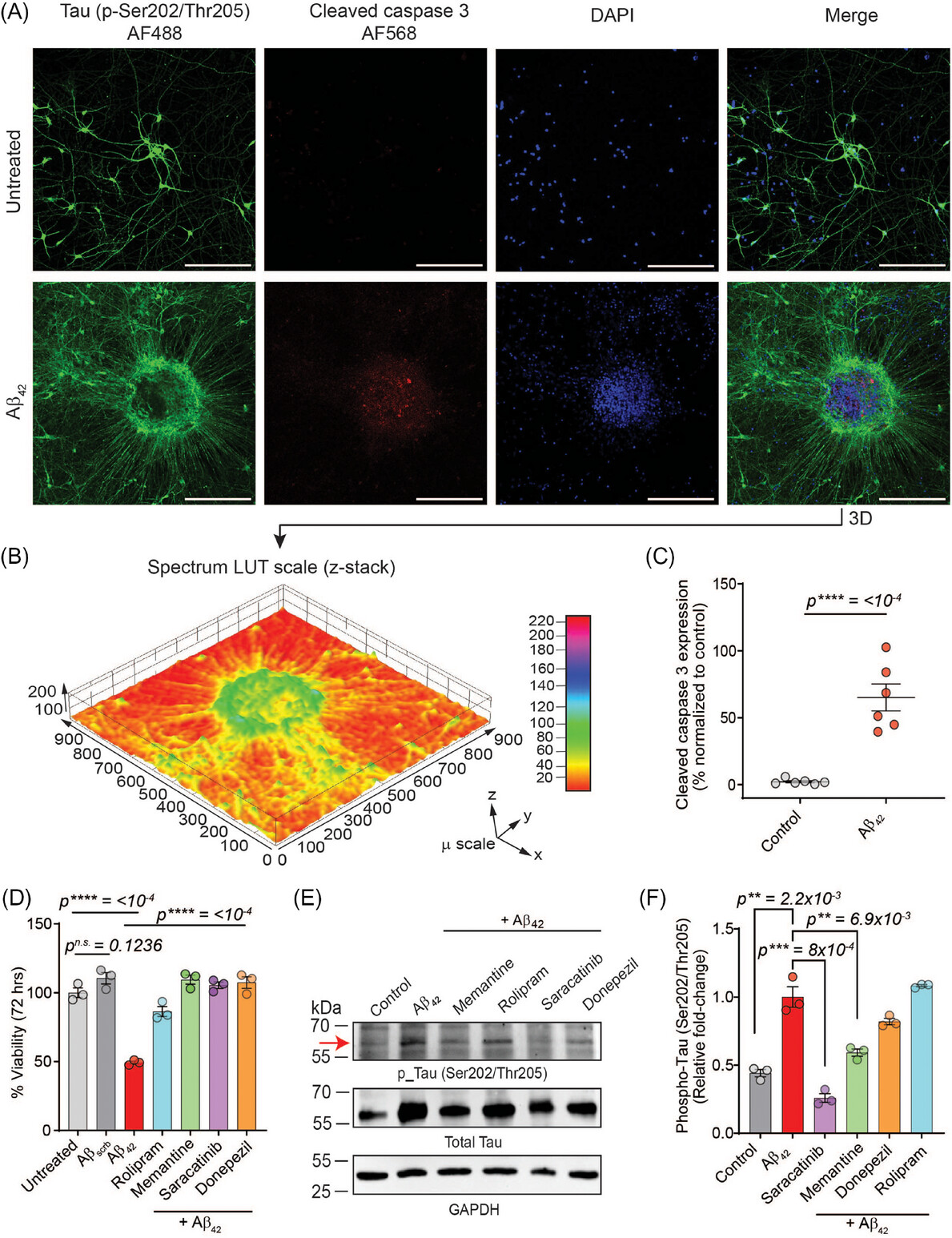
- Pathological Insights: Aβ42 induced synaptic damage, accelerated neuronal senescence, impaired mitochondrial potential, and increased reactive oxygen species (ROS), leading to oxidative stress and inflammation.
- Therapeutic Responses: Drugs such as memantine, rolipram, saracatinib, and donepezil improved neuronal function and viability, though they did not completely halt Aβ42-driven senescence.
The study underscores the potential of biomimetic MPS systems in translational research. By integrating clinically relevant neuronal function with proteome responses, this platform is poised to accelerate the discovery of novel neuroprotective compounds for AD and other neurological disorders.
“This marks a significant step forward in Alzheimer's research by providing a deeper understanding of AD pathophysiology in a human aged MPS platform. We are excited about the potential of this model to transform drug discovery in AD, providing hope for patients,” said J. Hickman, PhD, co-founder and Chief Scientist of Hesperos and Professor of Chemistry at UCF.
This research was supported by grants from the National Institutes of Health (R44TR001326, R44AG058330).
Research Article: doi.org/10.1002/alz.14044
Nanoscience Technology Center (NSTC) at the University of Central Florida (UCF)
The mission of the NSTC is to establish a cutting-edge research program in materials and nanotechnology, provide high quality training for students and facilitate the advance of innovations to solve real world technology challenges.
Hesperos, Inc.
Hesperos is a global contract research organization (CRO) providing drug development services using its Human-on-a-Chip® platform - the most advanced, multi-organ microphysiological systems available today. https://hesperosinc.com
“This marks a significant step forward in Alzheimer's research by providing a deeper understanding of AD pathophysiology in a human aged MPS platform. We are excited about the potential of this model to transform drug discovery in AD, providing hope for patients.”
- J. Hickman, PhD, co-founder and Chief Scientist of Hesperos and Professor of Chemistry at UCF.