Available Models
Select from our standard models or customize your own
2-5 Organ+
Reconfigure the platform to include the relevant organ tissues for your application.
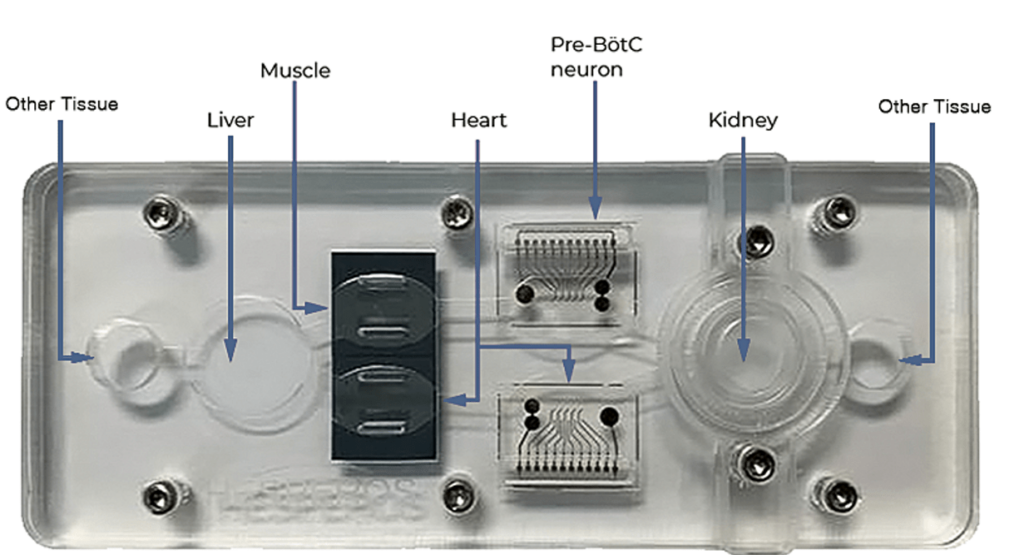
Custom Models
The platform can be easily configured to include virtually any organ, barrier tissue, or tumor. Schedule a webinar with our research team to determine the model that will best fit.

Blood-brain barrier (BBB model)
Our BBB model, composed of iPSC-derived cells, has been extensively characterized for tight junction-mediated barrier formation and paracellular (passive) and transcellular (active) transport mechanisms [6]. This module is well-suited for investigating the transport rates, transport mechanisms and barrier effects of novel chemicals and biologics.

Gastrointestinal tract (GI tract model)
Using either iPSC-derived enterocytes, or immortalized patient biopsy intestinal epithelial cells (hiECs), the absorption and first-pass metabolism characteristics of a novel compound can be determined. Our hiEC model has been characterized for CYP activity and barrier formation [7]

Kidney renal proximal tubule (RPT model)
Using primary renal proximal tubule cells, the tubular reabsorption or secretion profile of a novel compound can be determined for excretion purposes. Additional data regarding the kidney safety profile of a drug can be collected in the form of barrier integrity changes (noninvasive TEER measurements) and release of the biomarker kidney injury marker-1 (KIM-1).

Skin
Using Strat-M® membranes, a synthetic human skin module, integrated into our multi-organ systems, we can determine the transdermal diffusion of novel compounds applied topically and monitor the effects of the absorbed drugs on organ physiology in the system. We have characterized a heart-liver-skin HoaC model for the effects of topically-applied drugs including hydrocortisone, ketoconazole and diclofenac.
Monocytes / Macrophages
Monocytes can be added to the recirculating medium in our multi-organ systems. These cells (THP-1 monocytes) have been characterized in systems for expression of the lineage markers CCR5, CD14 and CD16 as well as markers of activation including CD11b, CD69 and CD86 following exposure to lipopolysaccharide (LPS) and interferon-gamma (IFN-γ). Additionally, following exposure to LPS and IFN-γ the monocytes have been shown to differentiation into macrophages and adhere to damaged tissue modules. Differential cytokine release profiles have also been characterized in the context inactivated, tissue damage-activated and cytokine storm activated conditions.
Pharmacokinetic / pharmacodynamic modeling (CFD modeling)
Our recirculating medium allows for complex pharmacokinetic profiles of compounds and metabolic products through absorption, distribution, metabolism, and elimination (ADME) depending on the organ systems incorporated. We have extensive experience coupling HPLC-MS data with modeling of these systems through computational fluid dynamics (CFD) and other numerical methods to produce pharmacokinetic profiles both in different medium compartments and accumulated in the cells. Coupled with our functional measurements, we have the capabilities to generate Pharmacokinetic-Pharmacodynamic (PK-PD) relationships [2].
High-performance liquid chromatography - mass spectroscopy (HPLC-MS)
Additional Organ Modules and Services
Using our Agilent Technologies HPLC-MS, we can quantify the concentration of drug in systems at any point during an experiment. Additionally, in any liver-containing HoaC system, we can determine concurrent depletion of parent compound and metabolite generation.
References
- Stancescu, M., et al., A phenotypic in vitro model for the main determinants of human whole heart function. Biomaterials, 2015. 60: p. 20-30.
- Oleaga, C., et al., Investigation of the effect of hepatic metabolism on off-target cardiotoxicity in a multi-organ human-on-a-chip system. Biomaterials, 2018. 182: p. 176-190.
- Oleaga, C., et al., Human-on-a-Chip Systems: Long-Term Electrical and Mechanical Function Monitoring of a Human-on-a-Chip System (Adv. Funct. Mater. 8/2019). Advanced Functional Materials, 2019. 29(8): p. 1970049.
- Santhanam, N., et al., Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics. Biomaterials, 2018. 166: p. 64-78.
- Oleaga, C., et al., Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Scientific Reports, 2016. 6: p. 20030.
- Wang, Y.I., H.E. Abaci, and M.L. Shuler, Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnology and Bioengineering, 2017. 114(1): p. 184-194.
- Chen, H.J., P. Miller, and M.L. Shuler, A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab on a Chip, 2018. 18(14): p. 2036-2046.
Explore
Latest News
Get in Touch
Hesperos, Inc.
12501 Research Pkwy, Suite 100
Orlando, FL 32826
(407) 900-5915
